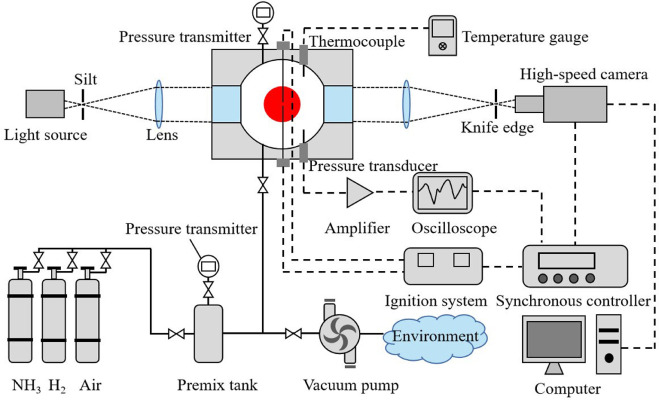
Laminar burning velocity, Markstein length, and cellular instability of spherically propagating NH3/H2/Air premixed flames at moderate pressures
Huizhen Li, Huahua Xiao*, Jinhua Sun
Abstract:
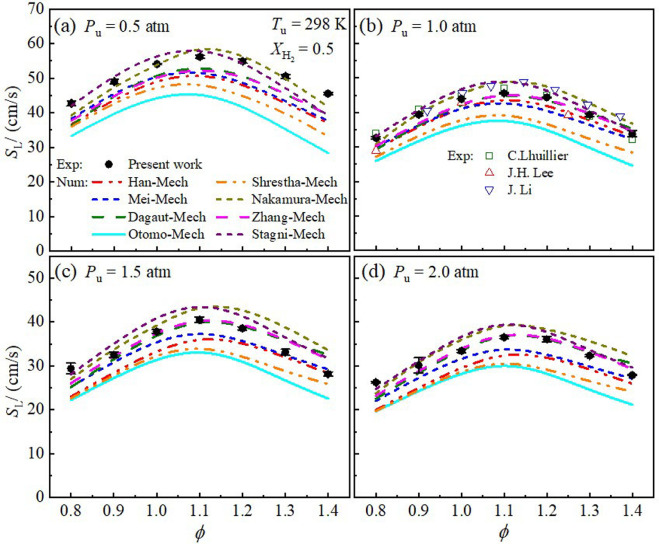
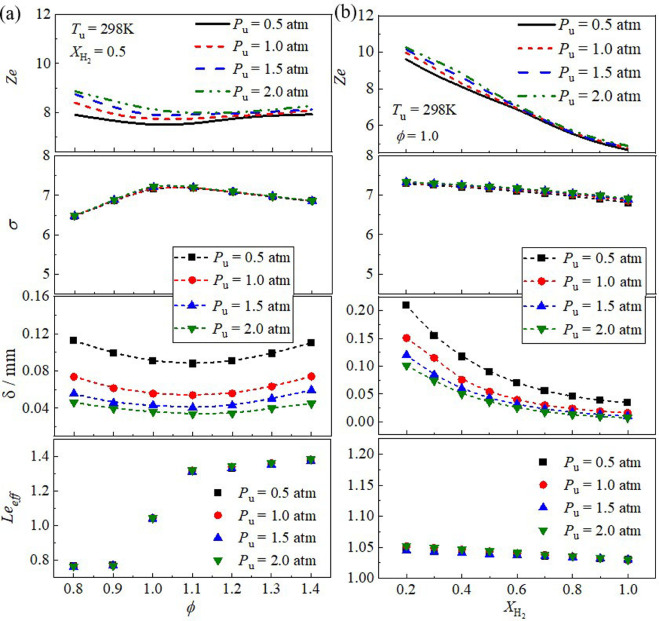
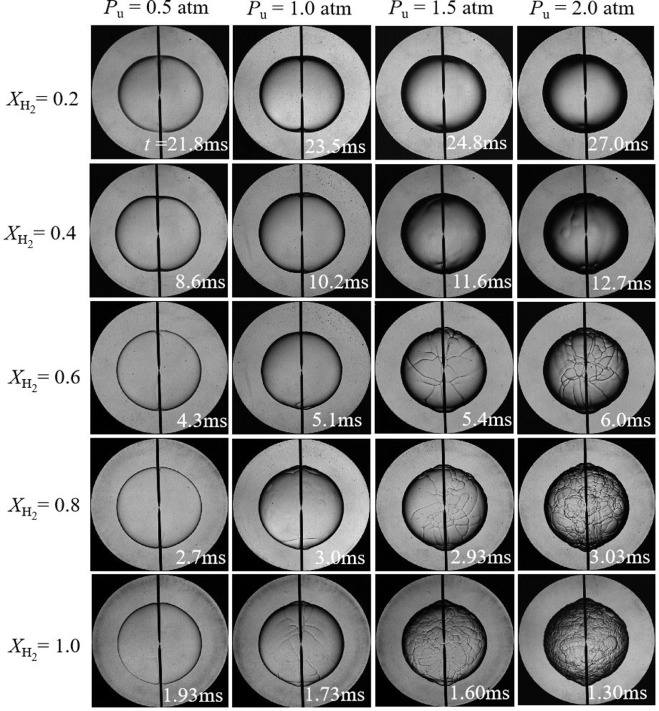
Blending hydrogen (H2) into ammonia (NH3) as a carbon-free fuel has attracted increased attention. However, fundamental experimental data on laminar combustion characteristics of NH3/H2/air mixtures are rather rare for moderate pressure (0.5 - 2.0 atm). In this work, experiments of laminar flame properties of NH3/H2/air mixtures were performed using the spherical flame propagation method in a constant-volume chamber at initial temperature Tu = 298 K. Numerical simulations with various kinetics mechanisms were conducted and compared to the experiments. Effects of equivalence ratio (ϕ = 0.8 - 1.4), hydrogen ratio ( XH2= 0.2 - 1.0), and initial pressure (Pu = 0.5 - 2.0 atm) were scrutinized. The results show that laminar burning velocity increases with increasing hydrogen ratio due to the enhancement of both thermal and chemical kinetics effects. The influence of pressure on laminar burning velocity depends on hydrogen concentration, i.e., a negative effect for XH2< 0.8, while a positive effect for pure H2. In particular, the laminar burning velocity is insensitive to pressure variation at XH2= 0.9. The Markstein length increases monotonically with the increase of equivalence ratio, and decreases with initial pressure from 0.5 to 1.5 atm, but slightly increases with increasing the initial pressure from 1.5 to 2.0 atm. Flame morphology shows that the NH3-H2 flames suffer from cellular instabilities except Pu = 0.5 atm. Cellular instability arises at lean burn because of the combined effects of thermal-diffusive and hydrodynamic instabilities. Increasing hydrogen ratio or initial pressure can promote the global flame instability caused by hydrodynamic destabilizing mode. In addition, the simulations show considerable discrepancies between different reaction mechanisms and experiments for certain ranges of initial conditions, and thus the current measurements can be useful for improving and validating kinetics mechanisms.
Figures: